As part of my ongoing series, I review various local clinics on their stem cell offerings so that you can get the real skinny on what they’re offering. This morning is a review of the Keystone Health and Wellness Center in Greenwood Village. Let’s dive in.
What is Keystone Health and Wellness Center?
Keystone Health and Wellness Center in Greenwood Village, CO is a chiropractic clinic with a nurse and no physicians working at the clinic. The clinic states that it offers chiropractic, physical therapy, aesthetic, and stem cell services. It’s that last part that we’ll review here.
What Type of “Stem Cells” Does Keystone Health and Wellness Center Offer?
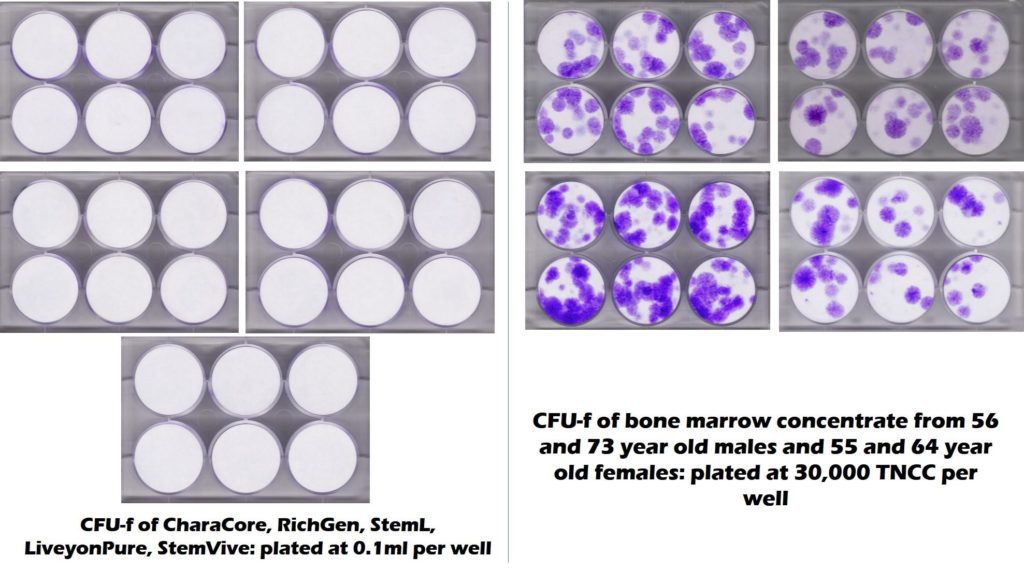
On its website, Keystone says that it offers “umbilical cord stem cells”. This is where we hit the first snag in this review, as the only umbilical cord stem cells that the clinic could be offering would be those registered as 361 donor tissues with the FDA. These products are sourced from umbilical cords and the FDA has issued public warnings about the practice of using these products to treat various illnesses (1). More importantly, multiple university and third-party labs have tested these and other FDA registered donor birth tissues and found that NONE contains any living and viable stem cells (2-4). In fact, the CSU Translational Medicine Institute just tested 5 commonly used umbilical cord products that all claimed live stem cells and those results are below:
On the left, you see the test results of 5 umbilical cord products showing all white. In this test, living and functional stem cells will stain purple, like the plates you see on the right. In this test, the plates on the right are from middle-aged and elderly bone marrow and clearly show stem cells. Hence, the umbilical cord products had no stem cells while middle-aged and elderly had loads of stem cells. Hence, the assertion that Keystone is using an umbilical cord “stem cell” product is not supported by the scientific data sourced from four separate labs.
Who Is Injecting the Non-Viable Umbilical Cord Product?
As you can see above, what Keystone is injecting is not a viable and functional stem cell product, so we’ll move onto who is injecting this product. Is this an expert physician specialist with additional training on how to perform interventional orthobiologics? No, this is a nurse who has generally about 1/3 of the training of the average physician specialist.
The X-rays Showing Efficacy
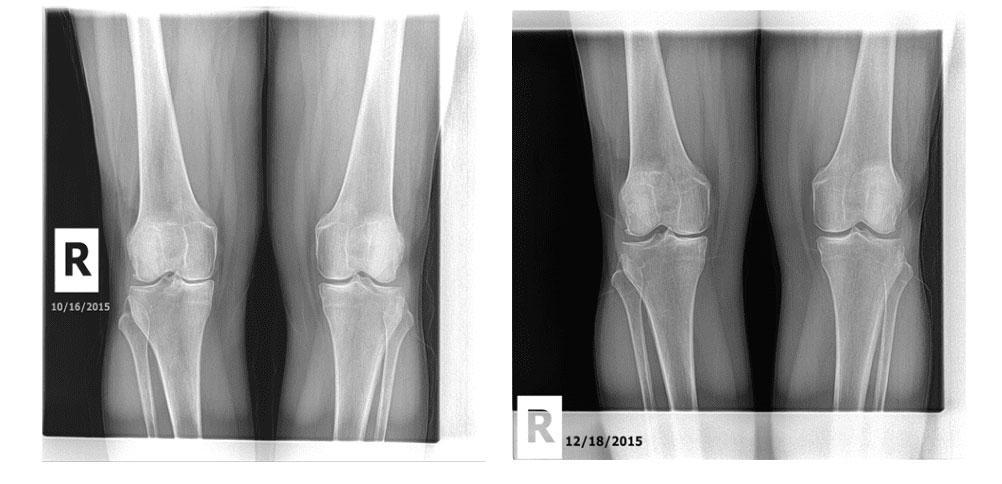
Keystone Health and Wellness show two pairs of x-rays as “proof” of the efficacy of its non-viable “stem cell” therapy. First up is this set of x-rays:
First, these x-rays state that they were taken in 2015. A review of the Internet Archive demonstrates that in 2015 there was nothing about regenerative medicine on the Keystone website nor was there a nurse working there who could have provided the injections. Hence, it would seem unlikely that these x-rays were taken at Keystone.
More importantly, the x-rays claim to show that the width of the knee joint has increased in an arthritic knee. The implication is that this happened due to cartilage regeneration. However, a close examination of the films shows that the angle of the x-ray beam is different between these two films, which is a problem of measurement that would make the image look like it had a wider joint without anything actually having changed. To learn more about how that works, see my video below:
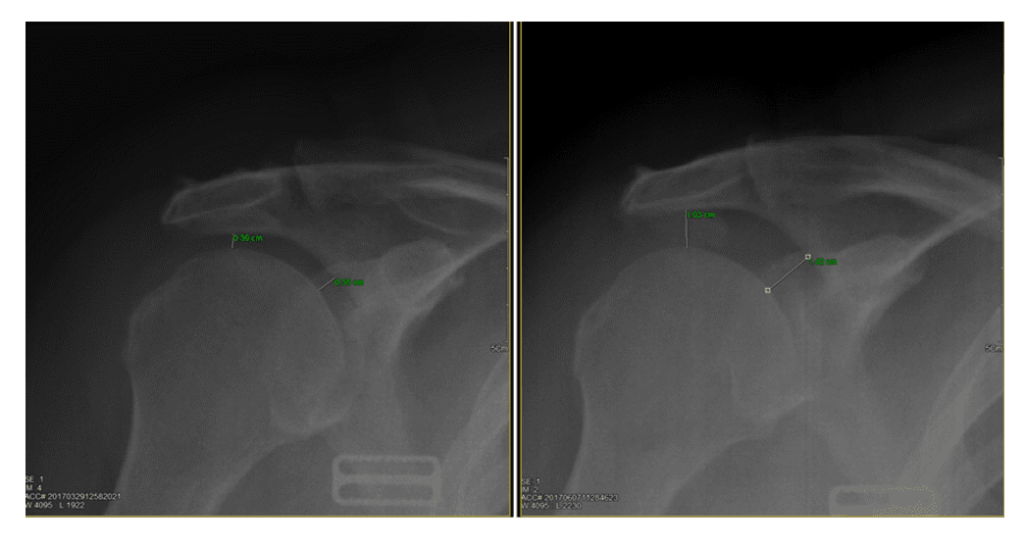
Next up are these x-rays, which regrettably, in my opinion, are an even bigger problem:
The shoulder x-ray on the left is not taken to see the main joint space well, so the joint space measurements are not going to be very accurate. However, the “After” stem cell therapy image is a huge problem. Why? It shows a dramatically worsened shoulder. In those films, the main shoulder joint has become unstable, so the increase in measurements is a bad thing and not a good thing.
Danger Will Robinson…
Up until this point the Keystone Health and Wellness website just had lots of clearly inaccurate stuff. However, then I chanced upon some things that could indicate danger. Let’s dive in.
The following appeared on their website:
“We use Umbilical Cord Stem Cells as they come from healthy mothers delivering healthy babies. We consider them the best for quite a few reasons.
- Umbilical Cord Stem Cells are undifferentiated cells which means no potential allergic reactions.
- Umbilical Cord Stem Cells are immune privileged which means anyone can be treated.
- Umbilical Cord Stem Cells are simple, fast, and safe through a quick direct injection or an IV fusion.”
Why is this a problem? First, while it’s true that umbilical cord stem cells if they were isolated, culture-expanded, and healthy, are immune privileged, that’s NOT what Keystone is using. Instead, they using a non-viable nucleated cell umbilical cord product. That means that these products contain a mix of cells, most of which are capable of causing an immune reaction in the patient to which they’re injected.
For example, it’s quite clear that when umbilical cord blood products are delivered IV to treat a specific type of rare pediatric cancer that they need to be matched to the patient (5,6). The greater the mismatch, the more likely that a serious disease called Graft vs.Host Disease can occur. This can be as simple as a rash to as problematic as organ failure. To prevent this, HLA matching tests are required of both the product and the patient BEFORE the infusion occurs. Hence, none of these statements above are accurate when vetted against the published science.
The upshot? Keystone Health and Wellness isn’t much different than other chiropractic clinics offering “stem cells” that I’ve reviewed. The “stem cell” source (umbilical cords) tells us that they are not using viable and functional stem cells. The provider performing the procedure is not a physician specialist but a nurse. The x-ray evidence doesn’t show the cartilage regeneration that is implied. However, the most concerning finding is the statement that the clinic is injecting umbilical cord cells without any type of matching the product to the patient which requires a series of sophisticated blood tests before the infusion.
Related:
Regenerative Medicine of the Rockies Review
Denver Regenerative Medicine Review
Colorado Medical Solutions Review
_______________________________
References:
(1) U.S. FDA. FDA sends warning to companies for offering unapproved umbilical cord blood products that may put patients at risk. Accessed 12/06/2019. https://www.fda.gov/news-events/press-announcements/fda-sends-warning-companies-offering-unapproved-umbilical-cord-blood-products-may-put-patients-risk
(2) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(3) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(4) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(5) Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood Jul 2014, 124 (3) 363-373; DOI: 10.1182/blood-2014-01-514786
(6) Lee SJ. Classification systems for chronic graft-versus-host disease. Blood Jan 2017, 129 (1) 30-37; DOI: 10.1182/blood-2016-07-686642