One of the most common questions I get asked every day in the clinic is what are my instructions for physical therapy after stem cell injections? As a physician who was the first in many cases to perform many common orthopedic stem cell injection procedures, I’ve had a decade and a half to figure out how to approach this issue. Here are my recommendations, which you might think of as a post stem cell protocol for physical therapy.
What is Stem Cell Therapy?
First, before I get into physical therapy after stem cell injections, it’s important to note what is and isn’t stem cell therapy. There are three common procedures being offered that are being called stem cell therapy, but only two actually have stem cells:
- Bone marrow concentrate (BMC or BMAC)
- Micronized adipose tissue (Lipogems)
- Birth tissue products like amniotic or umbilical cord tissue
Bone marrow concentrate is an autologous (from the same patient) treatment that removes the stem cell fraction from a bone marrow aspirate which is usually taken from the back of the hip area (PSIS). The treatment contains many cells including mesenchymal stem cells, hematopoietic stem cells, and many others. These cells are freely available to directly act on the tissues where they’re injected. This is the procedure with the most robust research showing that stem cells can help orthopedic conditions (4-8).
Micronized adipose tissue is also autologous and is created from the product of a mini-lipo-suction procedure. The fat is then run through a grate which chops it into fine pieces. The initial thought was that this liberated stem cells, but research from our lab has shown that this really doesn’t commonly occur so that the mesenchymal stem cells in this therapy are still locked in collagen. Hence, any effects that this type of treatment has on tissues would be through the stem cells indirectly acting on tissue through chemicals they excrete (also called a paracrine effect). There is a mounting evidence base that this type of therapy works about as well as bone marrow concentrate for common orthopedic conditions like knee arthritis (9).
Birth tissue products like umbilical cord blood, or Wharton’s Jelly, or amniotic membrane or fluid are often advertised by clinics as products containing millions of young and viable stem cells, however, this isn’t really the case. Several studies have shown that these tissues have no live and viable stem cell content and are mostly dead tissue (1-3). They do have growth factors and cytokines that may help stimulate tissue repair. However, there is very limited to no evidence of their efficacy when used to treat common orthopedic conditions.
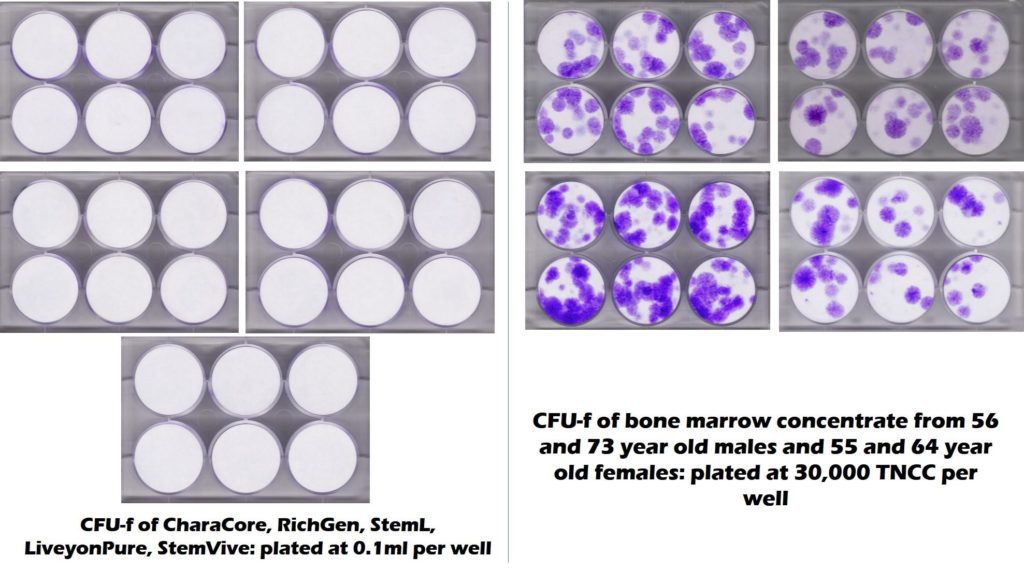
To get a visual sense of how many mesenchymal stem cells these commercially available birth tissues have versus bone marrow, the CFU-f plates below show stem cell content as purple colonies (the circular dots). On the right is middle-aged and elderly bone marrow and on the left are five commonly used umbilical cord products showing no stem cell colonies.
How Is Physical Therapy for Stem Cell Injection Procedures Different?
If stem cells are used as part of orthopedic surgery, there is generally no difference between the usual post-surgical physical therapy recommendations and the standard protocols. However, where there are big differences is in physical therapy after stem cell injections. Why? Unlike orthopedic surgery, most stem cell injections cause minimal downtime and disability. Meaning much of post-surgical rehab is focused on getting the patient back to where they were before surgery, as the surgery often causes severe pain and is often accompanied by weight-bearing or other restrictions or short-term lack of function. However, physical therapy after stem cell injections should be focused on remediating the structural and strength deficits that caused the problem being treated. Let’s dig in.
To better understand the rationale for various recommendations below, watch my short video on the phases of healing after regenerative medicine procedures (this is focused on ligaments, but can also apply to many other tissues):
Many of these same restrictions also apply to platelet-rich plasma injections.
Phase 1-Immediate Post Injection Care-0-2 Weeks
In this phase, the stem cell injection will likely have caused a short-term inflammatory response lasting 3-7 days. Cells are being recruited to the area. These patients break into two categories:
- Activity restriction
- Activity as tolerated
Activity Restriction-These are patients with unstable tissues that still need to heal and where activity could harm that healing. As one example, a patient with an unstable fracture treated with a fluoroscopically guided intraosseous (into the bone) injection of bone marrow concentrate. This patient would obviously be non-weight bearing on that body part for at least 6-8 weeks. Or if a patient has a significant rotator cuff tear with an ultrasound-guided injection of BMC, they may be in a sling or told to perform only passive range of motion during this time.
Activity as Tolerated-In this category would be patients who have otherwise stable tissues. This is a time for rest and reduction of activities, but the jumping-off point for a return to light activities. For example, if the shoulder patient above had just partial rotator cuff tears, then the patient could resume light activities such as lighter weights in the gym while listening to their body (only 25% of their usual gym weights). We use the rubric of not allowing the pain to get above a 2-3/10 during activity. If it rises higher, then the patient is instructed to back off. Another example is a spinal injection of stem cells or platelet-rich plasma around nerves (epidural), into damaged facets, or ligaments, or into atrophied muscles. These patients can also return to light activities. Or a patient with knee arthritis who had micro fragmented fat injected into the knee can go on short to medium duration walks during this time period.
Phase 2-Post Injection Care-2-6 Weeks
At this point, the acute phase of healing is over and in some types of tissues like ligaments and tendons there will be new extra-cellular matrix laid down by the stem cells. In other patients like those with knee arthritis, the cells are modulating inflammation and jump-starting a burnt-out repair response.
For activity restricted patients like our fracture case, full weight-bearing is still not permitted. For the more damaged rotator cuff tear patient, passive range of motion gives way to early active-assisted range of motion without weight. For example, using the fingers to crawl up a wall to promote abduction of the shoulder, not using the rotator cuff to move the arm.
For activity as tolerated patients, this is the time that they can advance to low weight, high repetition activities. For example, our partial rotator cuff tear patient may be able to advance from light Therabands to begin using light weights. As long as this is tolerated without pain, the patient can advance to light weights with higher reps for rotator cuff strengthening. However, this shouldn’t be more than 50% of their usual weights. For our spinal patient, they can return to most activities as long as those activities don’t cause a 2-3/10 pain or greater. Or for a patient with knee arthritis, they can also return to activities such as cycling and long-distance walking (up to 1-2 miles) as long as there is no significant pain.
For these higher activity patients without significant pain, this is the time for physical therapists to begin to address strength and function. Again these are those functional deficits that caused the problem being treated. For example, our rotator cuff patient may have had a protracted shoulder and a weak rotator cuff with poor neck stabilization.
Phase 3-Post Injection Care-6 Weeks to 3 Months
This is the time for tissue maturation. Loading here is a net positive for most patients, as it takes disorganized fibers or tissue and creates the appropriate matrix and fiber alignment for mature tissues.
For our activity restriction patients, this is usually the time that restrictions begin to be lifted. In the 2-month time frame, our unstable fracture patient can begin to load the area more. In the 8-week time frame, our complete rotator cuff tear patient can advance from passive assist range of motion to active range of motion and then by 8 weeks to light Therabands. By three months this same patient can advance to light weights and then by four to full weights in the gym.
For activity as tolerated patients, this is the time to open the throttle to full activities if they’re not already at that point. For example, our partial rotator cuff year patient should be back to 75% of normal weights in the gym by 2 months and 100% by three months. Our spinal patient should have already returned to all normal activities by 6-8 weeks. Our knee arthritis patient should advance from long walks to full activity as tolerated by 2-3 months. This is also the time that physical therapists can advance to addressing more resistant strength and structural deficits.
Phase 4-Post Injection Care-3+ Months
For activity restricted patients, this is usually the time to open the throttle to full activities or advance towards same. This is also the time that physical therapists working with restricted patients can advance their work on addressing strength and structural deficits.
The upshot? Physical therapy after stem cell injections for most patients is different than post-surgical rehab. Return to activity is oftentimes faster, hence the physical therapist is working on addressing the problems that caused the treated problem much more quickly. In a few patients who need activity restrictions, physical therapy routines don’t differ all that much from post-surgical rehab.
___________________________________________________
See Chris N.’s recovery journey after stem cell therapy
References:
1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(4) Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008 May-Jun;11(3):343-53. https://www.ncbi.nlm.nih.gov/pubmed/18523506
(5) Centeno CJ1, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells. Med Hypotheses. 2008 Dec;71(6):900-8. doi: 10.1016/j.mehy.2008.06.042
(6) Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int. 2014;2014:370621. doi:10.1155/2014/370621
(7) Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet Disord. 2015;16:258. Published 2015 Sep 18. doi:10.1186/s12891-015-0714-z
(8) Centeno C, Sheinkop M, Dodson E, et al. A specific protocol of autologous bone marrow concentrate and platelet products versus exercise therapy for symptomatic knee osteoarthritis: a randomized controlled trial with 2 year follow-up. J Transl Med. 2018;16(1):355. Published 2018 Dec 13. doi:10.1186/s12967-018-1736-8
(9) Mautner K1 Bowers R, Easley K, Fausel Z, Robinson R. Functional Outcomes Following Microfragmented Adipose Tissue Versus Bone Marrow Aspirate Concentrate Injections for Symptomatic Knee Osteoarthritis. Stem Cells Transl Med. 2019 Jul 21. doi: 10.1002/sctm.18-0285.
