What’s the success rate of stem cell therapy for hips? You would be surprised that while we can help many problems with orthobiologics, there are some hip problems that can’t be helped. So let’s dig in to make sure you don’t get scammed.
What Can Go Wrong in the Hips that Can Cause Pain?
There are a number of structures around the hip that can get injured or that can wear out. These include cartilage, bone, labrum, ligaments, and muscles. Common hip conditions that cause pain are:
- Hip arthritis
- Labral tears or degeneration
- Avascular necrosis or osteonecrosis
- Hip bursitis
- Hip tendinopathy
The most common of these conditions where patients often seek hip stem cell therapy and there is published data is arthritis. So let’s dig into that area to determine the success rate of stem cell treatment for hips.
Does Stem Cell Therapy Work for Hips?
There are publications on three different types of cell therapies:
- Bone Marrow Concentrate
- Culture Expanded Stem Cells
- Micro-fragmented Fat
Bone Marrow Concentrate
Bone marrow concentrate is created by taking bone marrow aspirate and concentrating the stem cell fraction. This is rich in several stem cell types. This is called bone marrow concentrate (BMC) which is then injected into the hip joint using imaging guidance.
Our group (Regenexx Procedure) published a large case series of almost 200 patients that showed good results, but not in patients who were older than 55 or had more severe arthritis (1). A small study looked at 15 hips with early arthritis treated with bone marrow concentrate and they did well for 6 months (2). A very tiny study of 4 patients showed good results for a very limited amount of follow-up (3.5 months) (3). Hence, BMC seems to work in patients with mild to moderate hip arthritis, but not more severe hip arthritis.
Culture Expanded Stem Cells
Culture expanded stem cells are those that are isolated from bone marrow and then grown in culture to higher numbers. The stem cells are present in higher concentration in these procedures. We published the largest safety trial including cultured stem cells used in hip arthritis (6). There is only one very small study on the use of culture-expanded stem cells where 10 patients with mild hip arthritis had good outcomes (4).
Microfragmented Fat
This is a treatment where fat tissue is taken via liposuction and then processed in a kit called “Lipogems”. This slices the fat into small pieces so that it can be injected. This is also called microfragmented fat or Mfat. Six patients with mild arthritis were treated with Mfat and again, they reported good improvements (5).
Birth Tissues And Exosomes
There is no research that shows that amniotic or umbilical cord “stem cells” can help hip arthritis. There is also no research that exosomes can help. In addition, these birth tissue therapies do not have living stem cells (7-9).
How Much Does Stem Cell Therapy Cost for Hips?
Most therapies are not covered by insurance, but Regenexx has secured reimbursement from many large self-insured employers. If you’re paying out of pocket, then the cost range is about 4K – 8K for same-day procedures. However, be careful, as the therapies on the lower end of that cost spectrum tend to be the ones where corners are cut. This can include using non-physicians to perform the procedures, fake stem cell procedures that don’t contain stem cells and performing the procedures in a chiropractic or alternative health practitioner’s office rather than in an actual operating room.
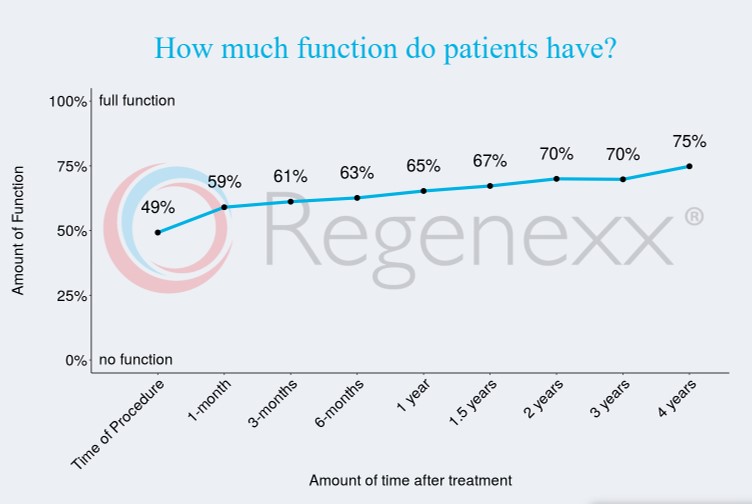
Success Rate of Stem Cell Therapy for Hips
Our published data online is likey the best indicator of how long these therapies last. This is 4-year data for the Regenexx procedure when used to treat hip arthritis:
This fits with my observation that in responders, the effects of the treatment last for 2-7 years.
The upshot? The success rate of stem cell therapy for hips is still being studied. Right now, high-dose bone marrow concentrate (Regenexx procedure) has the most data published. However, that’s sure to change as the field evolves.
___________________________________________________
References:
(1) Centeno et al., Efficacy and Safety of Bone Marrow Concentrate for Osteoarthritis of the Hip; Treatment Registry Results for 196 Patients J Stem Cell Res Ther 2014, 4:10. DOI: 10.4172/2157-7633.1000242
(2) Rodriguez-Fontan F, Piuzzi NS, Kraeutler MJ, Pascual-Garrido C. Early Clinical Outcomes of Intra-Articular Injections of Bone Marrow Aspirate Concentrate for the Treatment of Early Osteoarthritis of the Hip and Knee: A Cohort Study. PM R. 2018 Dec;10(12):1353-1359. doi: 10.1016/j.pmrj.2018.05.016.
(3) Darrow M, Shaw B, Darrow B, Wisz S. Short-Term Outcomes of Treatment of Hip Osteoarthritis With 4 Bone Marrow Concentrate Injections: A Case Series. Clin Med Insights Case Rep. 2018;11:1179547618791574. Published 2018 Aug 10. doi:10.1177/1179547618791574
(4) Mardones R, Jofré CM, Tobar L, Minguell JJ. Mesenchymal stem cell therapy in the treatment of hip osteoarthritis. J Hip Preserv Surg. 2017;4(2):159–163. Published 2017 Mar 19. doi:10.1093/jhps/hnx011
(5) Dall’Oca C, Breda S, Elena N, Valentini R, Samaila EM, Magnan B. Mesenchymal Stem Cells injection in hip osteoarthritis: preliminary results. Acta Biomed. 2019;90(1-S):75–80. Published 2019 Jan 10. doi:10.23750/abm.v90i1-S.8084
(6) Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016 Aug;40(8):1755-1765. doi: 10.1007/s00264-016-3162-y.
(7) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(8) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(9) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034